-
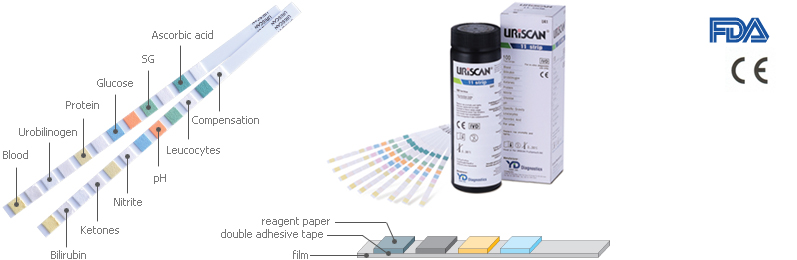
URiSCAN Strip

-
URiSCAN ACR Strip

-
URiSCAN Optima

-
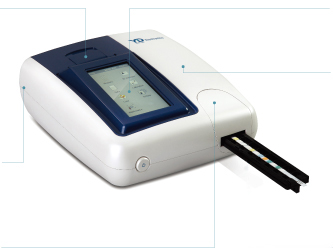
URiSCAN Pro

-
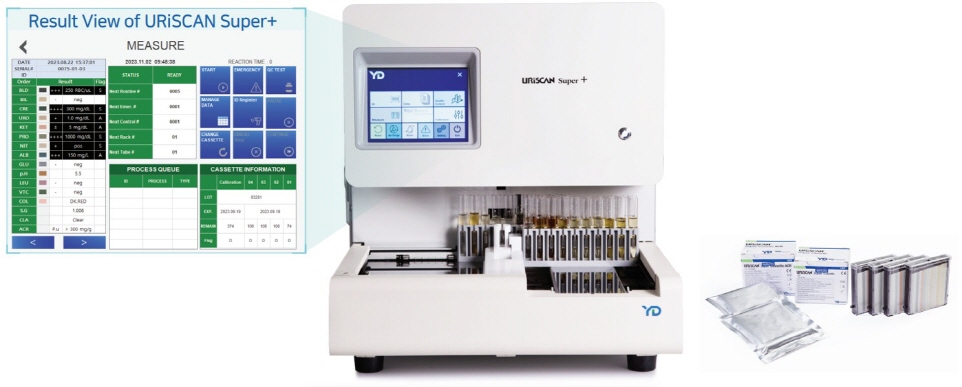
URiSCAN Super+

-
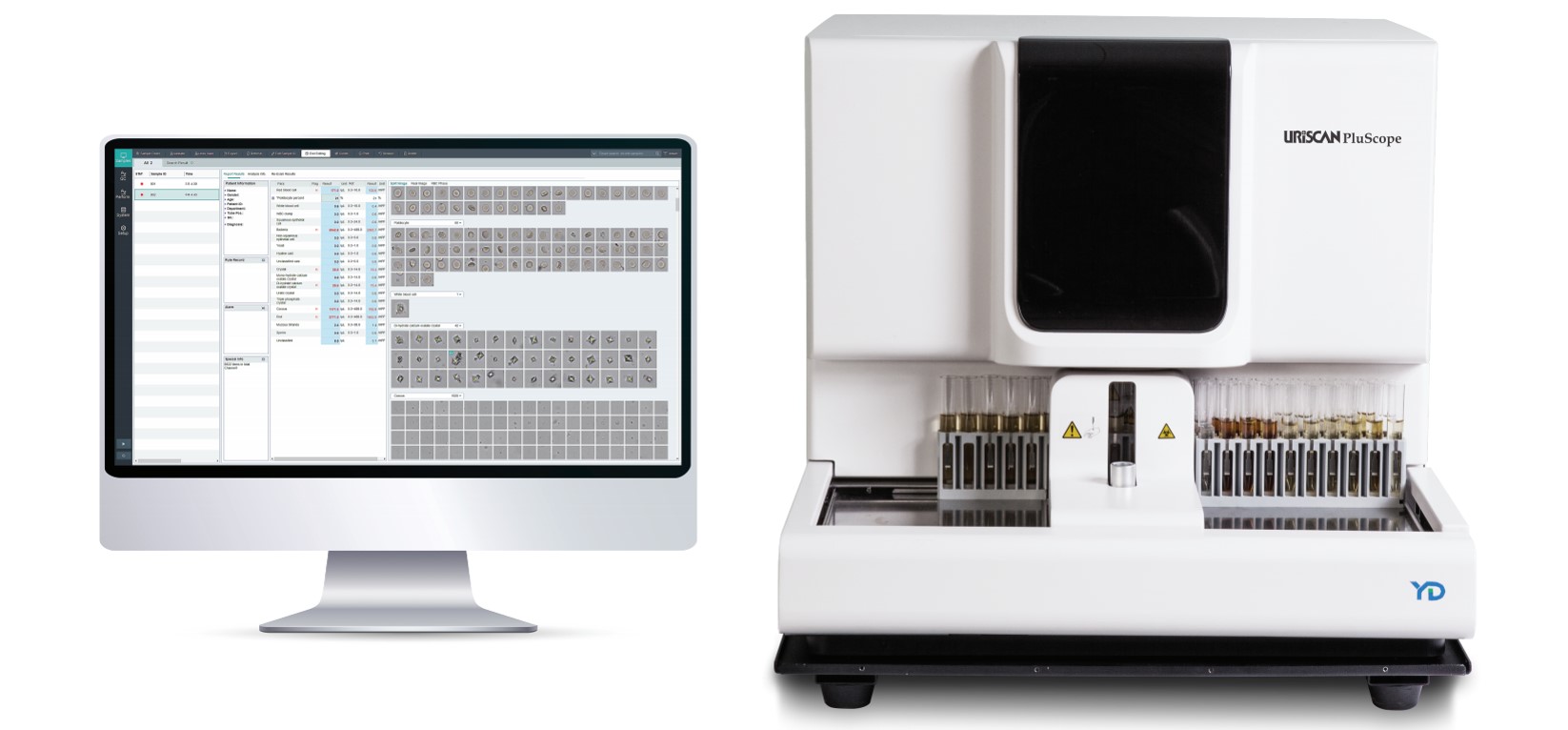
URiSCAN PluScope

-
URiTROL
 |


| FEATURES |
| ㆍ CE marked, FDA 510(K) approved and CLIA-Waived for professional use and self-test |
| ㆍ Four Configurations of Urine Strips : URiSCAN 2ACR, URiSCAN 10ACR, URiSCAN 11ACR strip and URiSCAN 13ACR Cassette |
| ㆍ Calculates Microalbumin to Creatinine Ratio |
| ㆍ Read with upgraded URiSCAN Optima, PRO and Super+ Urine Analyzer |
| URiSCAN Analyzers | |||||||||||||||||||
|
 |
||||||||||||||||||
| Product Line | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
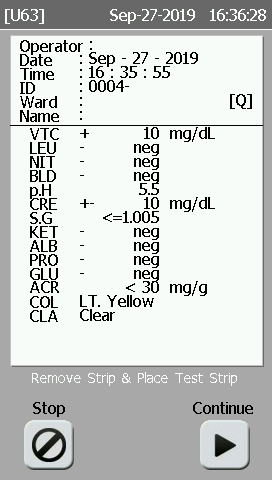
| Vt.C : Ascorbic Acid LEU : Leucocytes GLU : Glucose |
ALB : Microalbumin NIT : Nitrite PRO : Protein KET : Ketones |
URO : Urobilinogen CRE : Creatinine BIL : Bilirubin BLD : Blood |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||
| - Technical Specifications | |||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||
 |


ㆍPossible to connet to the PC or to the lab network system
ㆍOptional hand-held barcode scanner can be connected
ㆍExternal printer interface : pararell port connector

ㆍOnboard thermal printer for easy access to review test results
 |

ㆍAC/DC Adaptor : AC 100V-240V, 50/60Hz
ㆍURiSCAN PRO : DC 12V, 3.5A

ㆍ7″ Touch Screen TFT LCD
ㆍSoftware available in English, German, Spanish, Italian, Portuguess, Chinese, Russian and Korean
ㆍMemory capacity : 3,000 patient records,
1,000 control data and 30 calibration data
ㆍSimple calibration
ㆍEasy data management & various data printing format

ㆍHighest throughput : 720 tests per hour
ㆍClosed system : Allow to read only URiSCAN branded urine test strips
ㆍAutomatic adjustment for urine color
ㆍManual clarity input mode
Reflectance photometer :
CCD color image sensor and
LED for light source
| Technical Specifications | |||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||
 |
|
|
| Dimension(mm) | 551(W)X682(D)X575(H) |
| Weight | 57Kg |
| Test parameters | Blood, Creatinine, Bilirubin, Urobilinogen, Ketones, Protein, Nitrite, Micro Albumin, Glucose, pH, S.G., Leucocytes, Ascorbic Acid, Color, Clarity, A/C ratio by calculation |
| Measurement Principle | Test strip and color: Reflectance photometry (CCD image sensor ) Specific Gravity: Refractometer Clarity: Turbidimeter Memory capacity: more than 1,000,000 patient results, 500 control results Light source: LED |
| Reagent | URiSCAN Super Cassette (11 parameters) URiSCAN Super Cassette ACR (13parameters) |
| Reagent package | 400 samples at once(100’s X 4),continuous loading |
| Throughput | 200 T/h |
| Sample loading | 100 samples at once |
| External output | RS-232C,Ethernet, USB, PS/2, Parallel Communication |
| Power requirement | 110V–240V (50~60Hz), free voltage |
| External output | RS-232C,USB |
| Approval | KFDA, CE |

|
|
|
| Measurement Principle | Microscopic imaging analysis / Automated focus without focus liquid |
| Dimension(mm) | 610(W) X 780(D) X 550(H) |
| Weight | 51kg |
| Counting Chamber | 4 |
| Sample Loading | 10 tubes/rack, 10 racks onboard, constant loading by barcode scan |
| Throughput | Up to 120T/H |
| STAT | One STAT position and one probe clean position |
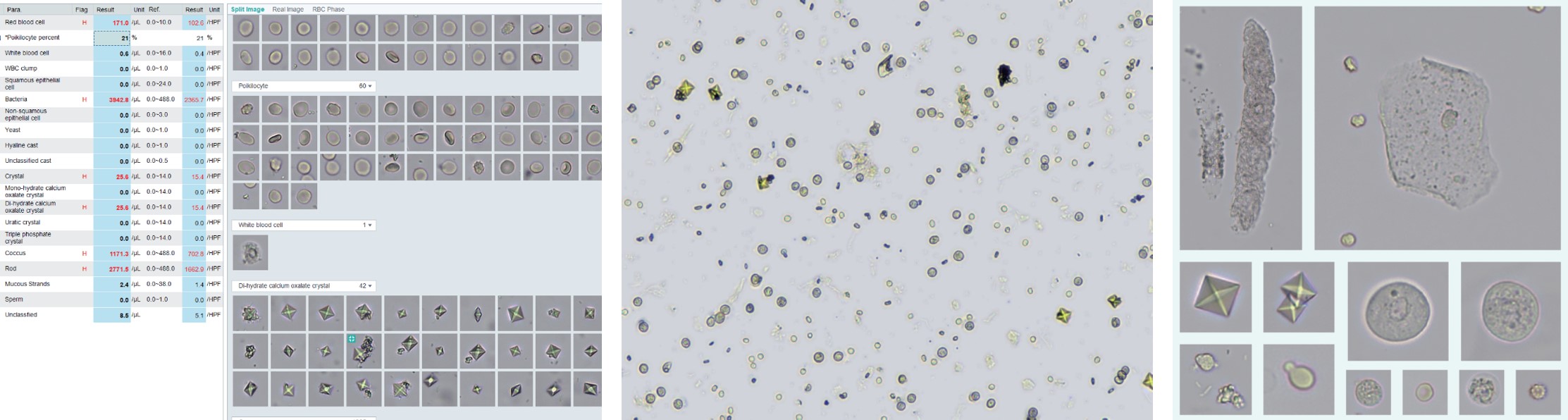
| Parameters | 18 reportable parameters(RBC, WBC, WBCC, BACT, BACTc, BACTr, YST, SEC, NEC, CRYS, Caoxm, Caoxd,
URA, TRP, HYAC, UNCC, MUC, SPRM), 9 RUO parameters of RBC phase, and 30 customized parameters |
| Reagent | Reagent B (for washing purpose) |
| Reporting format | International system of units in XX/µ, XX/HPF, or grade |
| Modularity | Yes |
| LIS | Bi-directional LIS, automatic identification of test mode |
| Approval | KFDA, CE |
|
|
|
|
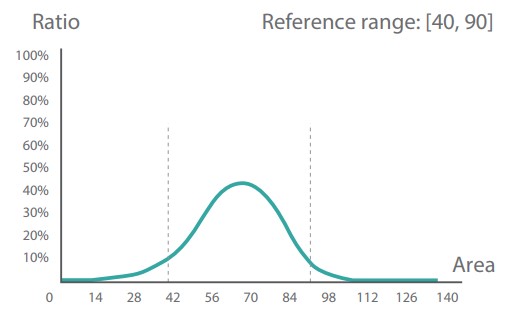
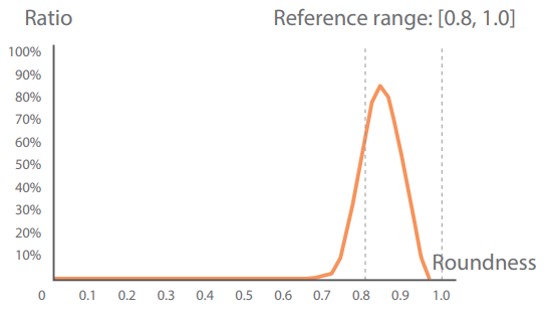
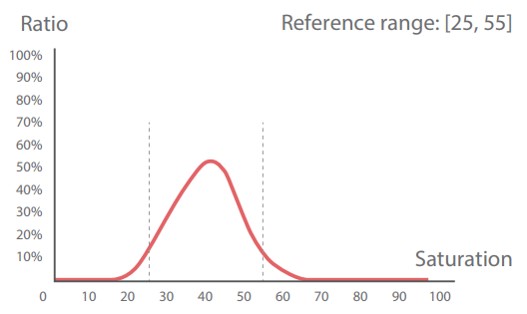
| RBC phase histograms showing the reference ranges and their clinical significance |
|
|
RBC size histogram | RBC shape histogram | RBC chroma histogram |
| Histogram |
|
|
|
| Unit of measurement | um^2 | Dimensionless | Dimensionless |
| Normocytic, Nor-RBC | 40-90 | 0.8-1.0 | 25-55 |
| Macrocyte, Mac-RBC | 50-85 | 0.75-1.0 | 25-55 |
| Microcyte, Mic-RBC | 20-55 | 0.8-1.0 | 35-60 |
| Crenocyte, Cre-RBC | 15-50 | 0.75-1.0 | 35-60 |
| Annular RBCs, Ann-RBC | 15-70 | 0.7-1.0 | 10-50 |
| Acanthocytes, Aca-RBC | 45-80 | 0.6-0.9 | 30-60 |
| Humped Spherocytes, Hpd-RBC | 45-85 | 0.55-0.8 | 25-55 |
| Jagged RBCs, Jag-RBC | 20-75 | 0.75-1.0 | 10-50 |
| Ghost RBCs, Gho-RBC | 10-50 | 0.75-1.0 | 5-18 |
| Fragmented RBC, Fra-RBC | 20-55 | 0.6-0.9 | 30-60 |
| Clinical significance |
Left shift indicates a decrease in size Right shift indicates an increase in size |
The roundness decreases when there is any abnormality |
Left shift indicates a decrease in hemoglobin concentration Right shift indicates an increase in hemoglobin concentration |
|
|
1.Automated urine chemistry analyzer |
|
2.Automated urine sediment analyzer |
|
||||
|
|
CAT no. | item | package |
|
CAT no. | item | package |
|
|
|
M8 | URiSCAN Super+ | Set |
|
USP | URiSCAN PluScope | Set (not including PC) |
|
|
|
U81 | URiSCAN Super cassette | 100’s X 4/inbox |
|
USP05-1 | Reagent B | 5LX2/box |
|
|
|
U80 | URiSCAN Super cassette ACR | 100’s X 4/inbox |
|
USP10 | Cleanser | 500mL |
|
|
|
Rinse | Rinse additive | 500mL |
|
|
|
|
|

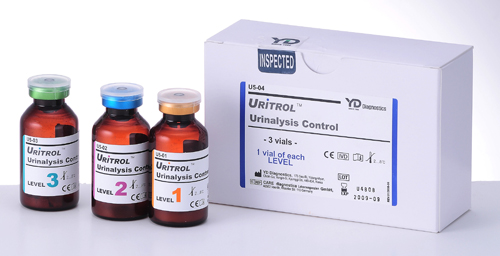
Urinalysis Control
| Product name | Item No. | Package | |
| URiTROL Liquid | Uritrol Liquid Level 1 | U5-11 | 15 ml × 3 |
| Uritrol Liquid Level 2 | U5-12 | 15 ml × 3 | |
| Uritrol Liquid 1, 2 | U5-13 | 15 ml × 2 | |
| URiTROL | Uritrol 1 | U5-01 | 10 ml × 3 |
| Uritrol 2 | U5-02 | 10 ml × 20 | |
| Uritrol 3 | U5-03 | 10 ml × 3 | |
| Uritrol 1, 2, 3 | U5-04 | 10 ml × 3 | |
 |
||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||
| URiTROL™ Liquid Urinalysis control, Dropper Style, is intended for use as an assayed quality control urine to monitor and maintain the accuracy and precision of the urine strip and the analyzers. | ||||||||||||||||||||||||||||||||||||||||||||||||
1) Unopened vial : it will the control is opened and stored at 2 ℃ to 8 ℃ 2) Opened vial : once the control is opened and stored tightly capped, all analyses will be stable for 2 weeks at room temperature (15 ℃ to 25 ℃) ※ Please do not store the control in a refrigerator after open. If the control stored temperature is changeable as like keeping in a refrigerator and room, the stability of control will be unstable. |
||||||||||||||||||||||||||||||||||||||||||||||||
|
ㆍ Liquid ready format ㆍ Maximum open vial stability ㆍ 24 months shelf life at 2 - 8 ℃ ㆍ High performance for most available urine test strips and strip system |
||||||||||||||||||||||||||||||||||||||||||||||||
| - Analyte Range_URiTROL Liquid | ||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| URiTROL™ Urinalysis control is intended to be used as an assayed quality control urine to monitor and maintain the accuracy and precision of the urine strips and the analyzers. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
1) Store at 2~8°C(refrigerator). 2) Once opened, all analytes will be stable for 10days (Level I & Level III), 24hours (Level II) or 20 immersions, when stored at 2~8°C, securely sealed. 3) You can extend the expiration date of URiTROL™ for 1 month after reconstitution as follows. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
ㆍ Lyophillzed product ㆍ Maximum Open Stability ㆍ 18 months shelf life at 2 - 8 ℃ ㆍ High performance especially for URiSCAN urine test strips and strip system |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| - Analyte Range_URiTROL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
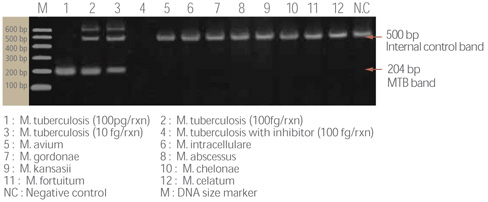
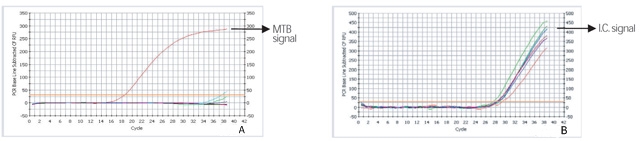
- Rapid and accurate detection only MTB among MTB complex
- Specimen : sputum, bronchial washed solution, body fluid, tissue, and cultured specimen
- High specificity and sensitivity with two-tube nested PCR
- All in one system : from DNA extraction to agarose gel electrophoresis of PCR product
- Internal control is included, determining existence of PCR inhibitor within specimens
- Sensitivity : detect up to 2 ~20 bacilli
- Specificity : detect specific target region for only MTB
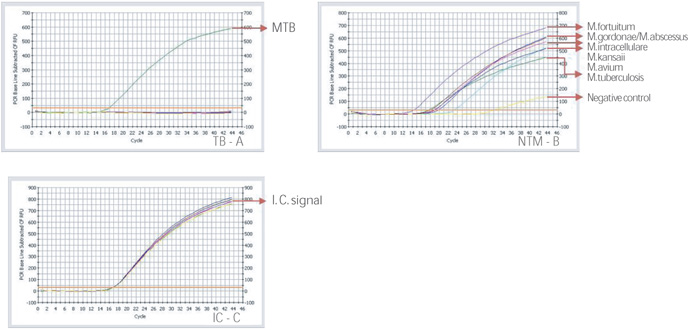
- Rapid and accurate detection and differentiates MTB and NTM at DNA level simultaneously
- Specimen : DNA of culture isolate
- High specificity and sensitivity by application multiplex and one-tube nested PCR
- All in one system : from DNA extraction to agarose gel electrophoresis of PCR product
- Internal control is included, determining existence of PCR inhibitor within specimens
- Sensitivity for MTB : detect up to 2~20 bacilli
- Sensitivity for NTM : detect up to 200 bacilli
- Specificity : no cross-reactivity between MTB and NTM
- Rapid and accurate detection
- Specimen : sputum, bronchial washed solution, body fluid, tissue, and cultured specimen
- High specificity and sensitivity
- Simple procedure and closed system
- Not required gel electrophoresis
- Minimized to cross or carry-over contamination in process - DNA extraction solution included
- Sensitivity : detect up to 2 ~20 bacilli
- Specificity : detect specific target region for only MTB
- Rapid and accurate detection
- Diverse clinical specimen : sputum, bronchial washed solution, body fluid, tissue, and cultured specimen
- High specificity and sensitivity
- Simple procedure and closed system
- Not required gel electrophoresis
- Minimized to cross or carry-over contamination in process - DNA extraction solution included
- Sensitivity for MTB : detect up to 2~20 bacilli
- Sensitivity for NTM : detect up to 200 bacilli
- Specificity : no cross-reactivity between MTB and NTM
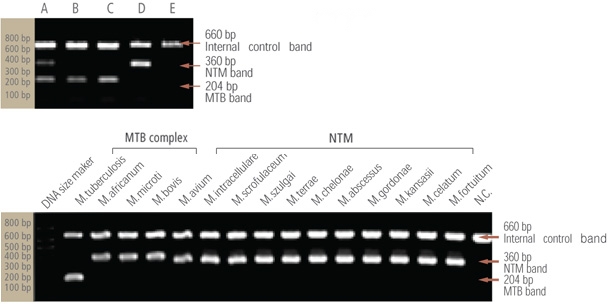
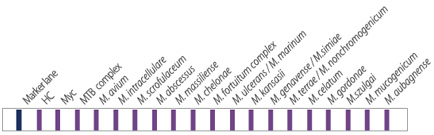
- Identification of 19 species of NTM
- Diverse clinical specimen : sputum, bronchial washed solution, body fluid, tissue, and cultured specimen
- High sensitivity and specificity by binding to species specific probe
- Rapid test within 2 hours after PCR reaction
- Useful for mixed or multiple infection of mycobacteria
- Sensitivity for NTM : detect up to 20 bacilli
- Specificity : identify MTB and 19 species of NTM (detect to over 100 species NTM)
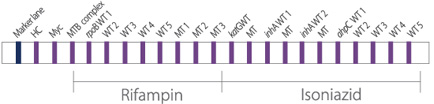
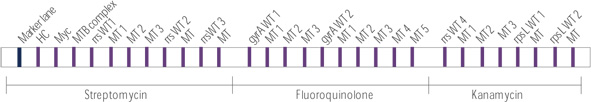
- Rapid and accurate detection of rifampin and isoniazid resistance of MTB
- Diverse clinical specimen : sputum, bronchial washed solution, body fluid, tissue, and cultured specimen (not required culture)
- High sensitivity and specificity by binding to target gene specific probe
- Rifampin target : rpoB
- Isoniazid target : katG, inhA, and ahpC intergenic region - Rapid test within 2 hours after PCR reaction
- Sensitivity : detect up to 20 bacilli
- Specificity : detect drug susceptibility for rifampin and isoniazid of MTB
- Rapid and accurate detection extensively drug-resistance (XDR) of MTB
- Diverse clinical specimen : sputum, bronchial washed solution, body fluid, tissue, and cultured specimen (not required culture)
- High sensitivity and specificity by binding to target gene specific probe
- Rapid test within 2 hours after PCR reaction
- Sensitivity for NTM : detect up to 20 bacilli
- Specificity : detect drug susceptibility for kanamycin, fluoroquinolone and streptomycin of MTB
-
PCR-based Assay for TB
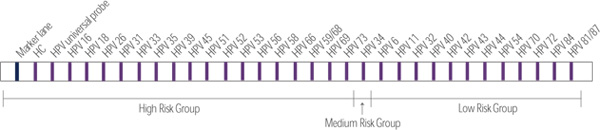
- Detect to HPV genotype of 18 species of high-risk, 1 species of medium-risk, and 13 species of low risk group at once
- High risk group : HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 66, 59, 68, 69,73
- Medium Risk group : HPV 34
- Low risk group : HPV 6, 11, 32, 40, 42, 43, 44, 54, 70, 72, 81, 84, 87 - Specimen : solid and liquid based specimen
- High sensitivity and specificity
- Rapid test within 2 hours after PCR reaction
- Full automation of REBA procedure and Result analysis
- Sensitivity : detects up to 100~1000 copies
- Specificity : No cross reactivity other type above this 32 genotypes
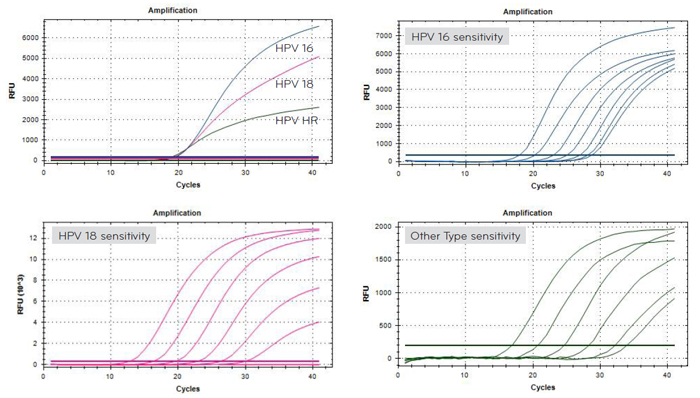
- Detect HPV 16, HPV 18, and 12 high-risk HPV
- High risk group : HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 - Specimen : swab, preservative solution
- High sensitivity and specificity
- Rapid test within 2 hours after PCR reaction
- Sensitivity : detects up to 100~1000 copies
- HPV 16 : detect up to 50copy/rxn
- HPV 18 : detect up to 50copy/rxn
- HPV HR : detect up to 102copy/rxn - Specificity : No cross-reactivity
- Specimen : swab, preservative solution, urine
- High sensitivity and specificity
- Rapid test within 2 hours after PCR reaction
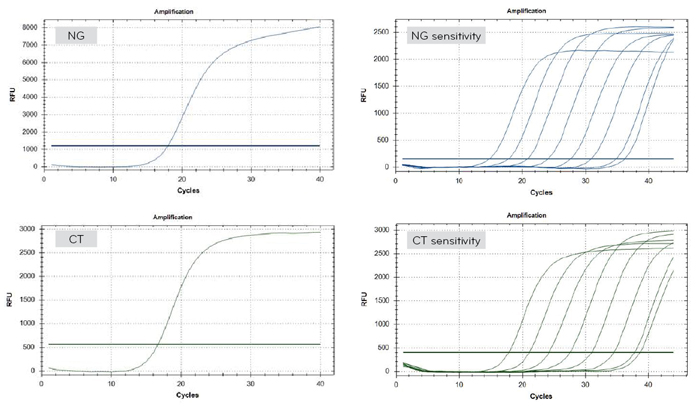
- Sensitivity :
- CT : detect up to 50copy/rxn
- NG : detect up to 102copy/rxn - Specificity : No cross-reactivity and interterence
- Specimen : swab, preservative solution, urine
- High sensitivity and specificity
- Rapid test within 2 hours after PCR reaction
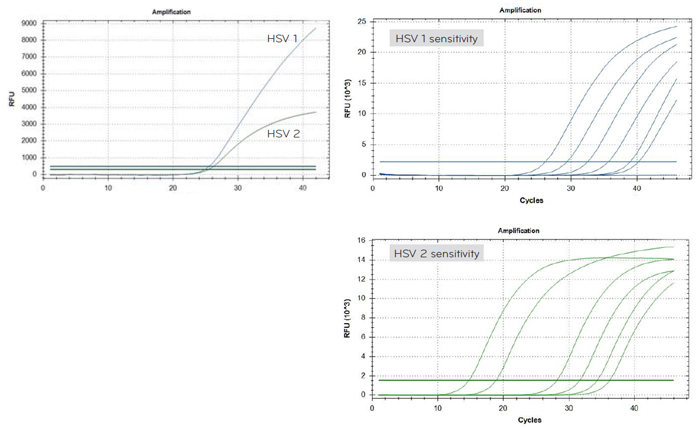
- Sensitivity :
- HSV 1 : detect up to 25copy/rxn
- HSV 2 : detect up to 6copy/rxn - Specificity : No cross-reactivity and interterence
-
Human Papilloma Virus(HPV) test

-
Device

-
Real-Time COVID-19
 |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| - Technical Specification |
 | |||||||||||||||||||||
|
|||||||||||||||||||||
 | ||||||||||||||||||||
|
||||||||||||||||||||
 | |||||||||||||||||||||
|
|||||||||||||||||||||
 | |||||||||||||||||||||
|
|||||||||||||||||||||
 | |||||||||||||||||||||
|
|||||||||||||||||||||
 | |||||||||||||||||||||
|
|||||||||||||||||||||
 | ||||||||||||||||||||
|
||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||
| - Technical Specification | |||||||||||||||||||||||||||||||||||
| MolecuTech REBA HPV-ID® | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| MolecuTech Real HPV 16/18/HR® | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| MolecuTech Real CT/NG® | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| MolecuTech Real HSV 1/2® | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Product Name | Cat No. | Information | e-catalog | |
 |
HybREAD480 | M-3 | Fully Automated REBA Processor & Analyzer |
 |
 |
DX32 | M-5 | Automated Nucleic Acid Purification System |
 |
| Information of Devices |
|---|

HybREAD480® is designed for full automation of strip assay from sample preparation to test results.
| ▣ HybREAD480® Features | |
|
|
| ㆍ Fully-automated precessor after PCR process | |
| ㆍ Automatic sample dispensing by robotic pipettor | |
| ㆍ Accurate analysis using CMOS camera | |
| ㆍ Maximum of 48 samples | |
| ㆍ Operating software with excellent ease of use | |
| ㆍ Built-in UV lamp for prevention of contamination | |
| ▣ HybREAD480® Structure |
|
| Technical Specifications | |||||||||||||||||||||
|
|
||||||||||||||||||||
| ▣ Applicable Reagent kit. | |
| ㆍREBA Myco-ID ㆍREBA MTB-MDR ㆍREBA MTB-XDR ㆍREBA HPV-ID |
|
| YD DX32 is a compact and reliable automated extraction platform in nucleic acid purification, based on the Magnetic Pillar technology, enabling successful delivery of extraction results of high quality nucleic acids for up to 32 samples from varied sample types. |
|
■ Kit Content
|
| ▣ Feature | |
|
ㆍ Ready-To-Go Solution
|
ㆍ Optimized Magnetic Pillar Technology
|
|
ㆍ Easy-To-Use User Interface
|
ㆍ Customizable Open System Platform
|
|
ㆍ Compact & light bench top instrument - fits in any laboratory |
|
|
ㆍ Perfect Match of Magnetic Particle Separation |
|
| Technical Specifications | |||||||||||||||||||||
|
|
||||||||||||||||||||
| Ordering Information | |||||||||||||||||||||||
|
|
||||||||||||||||||||||
|
|
| ㆍ Insert_MolecuTech Real-Time COVID-19 |
| ㆍ Fact Sheet for Healthcare Providers |
| ㆍ Fact Sheet for Patients |
| ㆍ Real-Time COVID-19 Rev.03 Leaflet |
| ㆍ MSDS |
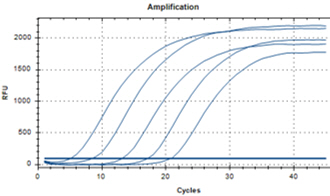
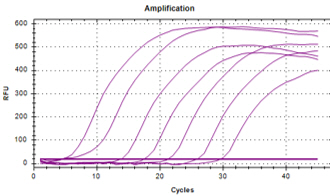
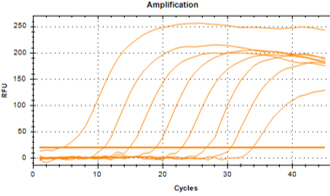
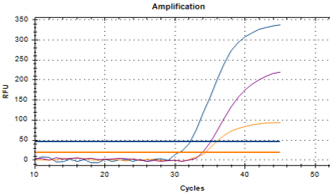
| YD’s MolecuTech® Real-Time COVID-19 test kit provides a solution to help laboratories address the urgent need for patient testing during the COVID-19 Pandemic. This multiplex real time PCR test kit consists of RdRP gene specific primer/probe to detect SARS-CoV-2 and E gene specific primer/probes for detecting all coronaviruses, and RNA extracted from respiratory specimens. |
 Features Features |
| ㆍDual target detection for RdRP and E gene |
| ㆍSpecific primer/probe mixture to increase its specificity |
| ㆍFast test results : 2 hours |
 Applicable Instruments Applicable Instruments |
| It is recommended to use either of the following diagnostic instruments: |
|
 Data Interpretation Data Interpretation |
|
 Contents Contents |
|
-
Tumor marker test

-
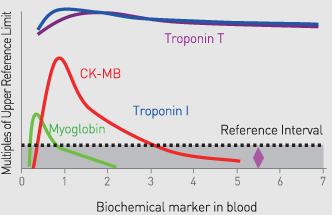
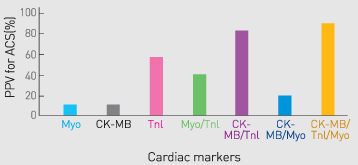
Cardiac marker test

|
||||||||||||||||||||||
| - Test kit for detection of Fecal Occult Blood | ||||||||||||||||||||||
 |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| - Technical Specifications of Tumor markers | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
-
Reagents for multiple auto-analyzer

-
Reagents for auto-analyzer & spectrophotometer

| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| - Clinical chemistry reagent | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| - LIPID PROFILE Automated assays for clinical analyzers | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The lipid profile is a group of tests comprising triglycerides, total cholesterol, HDL and LDL cholesterol. The lipid profile is used, together with other risk factors, to assess a person's risk of cardiovascular disease (CVD). It is very important to get the balance between the protective HDL and the destructive LDL right in order to reduce the risk of CVD. This can be achieved either through dietary and lifestyle changes or treatment with cholesterol reducing drugs called statins. Regular check-ups are necessary to establish risk and, if necessary, monitor treatment. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| - Clinical chemistry reagent | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| - LIPID PROFILE Automated assays for clinical analyzers | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The lipid profile is a group of tests comprising triglycerides, total cholesterol, HDL and LDL cholesterol. The lipid profile is used, together with other risk factors, to assess a person's risk of cardiovascular disease (CVD). It is very important to get the balance between the protective HDL and the destructive LDL right in order to reduce the risk of CVD. This can be achieved either through dietary and lifestyle changes or treatment with cholesterol reducing drugs called statins. Regular check-ups are necessary to establish risk and, if necessary, monitor treatment. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-
Papanicolau Stain

-
AFB Stain

-
Gram Stain

-
Wright Stain

Papanicolau Stain (Pap Stain)

|
|
|
| Papanicolaou stain is used for detection of early neoplastic changes in the uterine cervix. The primary purpose of obtaining a sample of cells from the cervix (Papanicolau smear) is to detect to cervical cancer, its precursors, and other abnormalities of the reproductive tract. | |
|
|
|
| EASYSTAIN Harris Hematoxylin and EASYSTAIN Gill’s-II Hematoxylin do not contain mercury oxide which can be very harmful to human body. | |
|
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|
||||||||||||||||||
|
||||||||||||||||||
AFB Stain

|
|
|
| This stain is used to differentiate the genus Mycobacterium from most other bacteria. | |
|
|
|
| Acid-fast cells (Acid Fast Bacilli) contain mycolic acid, a waxy material, in the cell walls. The Ziehl-Neelsen (ZN) method uses a primary stain of the lipid (fat) soluble carbolfuchsin, with steam heat acting as mordant to drive the stain into the cell. These cells resist decolorizing by acid alcohol. Nonacidfast cells will be decolorized. A counterstain of methylene blue will stain these decolorized cells. Acid-fast cells stain a red or pink, non-acid-fast cells stain blue. | |
|
|
||||||
|
||||||
|
|
||||||||||||||||||
|
||||||||||||||||||
Gram Stain

|
|
|
Gram-positive cell walls have a high affinity for the crystal violet stain because of their thicker peptidoglycan layer and lower lipid than that of Gram-negative cell walls. So, Grampositive cell walls retain crystal violet after alcohol rinsing, giving dark purple to blue coloring. The cell walls of Gram-negative bacteria have a very low affinity for the crystal violet stain, which is rinsed out by alcohol decolorizer. After counterstaining with Safranine solution, Gram-negative bacteria appear bright pink to red. |
|
|
|
|||||
|
|||||
|
|
|||||||||||||||||||||
|
|||||||||||||||||||||
YD Wright Stain

|
|
|
| The Wright Stain is used for staining of the human blood cells, especially differential cell count. | |
|
|
|
| Wright Stain is a solution which contains acidic dye, eosin, and alkaline dye, methylene blue, and dyes the acidic group or alkaline group containing a lot of components in each color. | |
|
|
|||||||||||||||||||
|
|||||||||||||||||||
|
|
|||||||||||||||||||||
|
|||||||||||||||||||||
- High Cellularity
- By adopting sedimentation and centrifuge method / No cell loss - Automation
- Total walk-away system : the world first processor with a built-in centrifuge - Select Mode
- Both GYN and Non-GYN tests are possible at the same time
- Various options are available to smear specific samples - Customization
- Easy adjustment of the parameter in accordance with laboratory requirement - Clarity
- Proper separation of mucus, inflammation cell and supernatants
- Cell shape and structure(morphology) are well maintained
- Clinically significant cells are evenly distributed for an easier reading - Barcode module (option)
- Preventing cross-contamination : EASYPREP system analyzes the barcodes on slides and sample(vial) for match. - HPV test
-
EASYPREP

 |

|
EASYPREP® System is a liquid-based cytology specimen collection, preservation and automated slide preparation system that produces standardized thinlayer slides for cervical cytology specimens and other non-gynecologic cytology specimens. | |||||||||||||||||||||||||||||||||||||||||||||
 | |||||||||||||||||||||||||||||||||||||||||||||
 Features Features | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
 Ancillary Test Ancillary Test | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
 Reference Reference | |||||||||||||||||||||||||||||||||||||||||||||
|
1. Cytological Evaluation and REBA HPV-ID HPV testing of Newly Developed Liquid-Based Cytology, EASYPREP : Comparison with SurePath. | |||||||||||||||||||||||||||||||||||||||||||||
|
2.Comparison of EASYPREP and SurePath in Thyroid Fine-Needle Aspiration. | |||||||||||||||||||||||||||||||||||||||||||||
 Technical Specifications Technical Specifications | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
 Product line. Product line. | |||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||
| Real-Time COVID-19 | |
|
 |




 Convenient to use for both of visual and analyzer
Convenient to use for both of visual and analyzer 


















 Rapid test time : 5 minutes
Rapid test time : 5 minutes